Written by Zack Jones, Developer, Iatric Systems
 We talk with many hospitals and healthcare IT vendors about medical device connectivity, and IHE is a topic that comes up often. However, there’s still a lot of uncertainty about what it’s for, why it’s needed, and what all the unfamiliar nomenclature means.
We talk with many hospitals and healthcare IT vendors about medical device connectivity, and IHE is a topic that comes up often. However, there’s still a lot of uncertainty about what it’s for, why it’s needed, and what all the unfamiliar nomenclature means.
If you want to learn about IHE quickly, register to attend an online webinar on Monday, November 9th at 2:00 p.m. ET, where guest speaker John J. Garguilo from the National Institute of Standards and Technology (NIST) will discuss how IHE standards are helping advance medical device integration in healthcare today.
{{cta('154ef42a-df1e-4844-a6b7-56c554e76fa8','justifycenter')}}
Below is a quick technical overview to help you understand the basics about IHE in the meantime:
What Is IHE, Anyway?
Integrating the Healthcare Enterprise (IHE) is an initiative by healthcare professionals and the industry to provide interoperability between disparate devices and systems. Products developed in accordance with IHE standards communicate with one another better, are easier to implement, and enable care providers to use information more effectively than relying on HL7 standards alone.
Why Does It Matter?
Since HL7 is already an established standard for connecting disparate systems, why do we need another one? There’s a saying in the HL7 world: if you’ve seen one HL7 spec, you’ve seen one HL7 spec. There are so many HL7 flavors, variations, and iterations that there’s no guarantee System A will be able to send a message to System B. IHE aims to bridge that gap, building on HL7 and other existing standards wherever it makes sense.
IHE simplifies medical device integration. IT professionals, clinical informaticists, and biomedical engineers should factor IHE compliance into any medical device related purchase. Increasingly, compliance with IHE standards is the key factor in being able to complete connectivity projects on-time and on-budget. If a vendor is closely following the IHE standard, hospitals and other vendors have confidence that they will be able to work together effectively, and bring a project to completion quickly.
How Is It Organized?
IHE is organized by domains, which are simply technical and operational categories. In each domain, users with clinical and operational experience identify integration and information sharing priorities, and vendors of relevant information systems develop standards-based solutions to address those priorities. These solutions address one or more Integration Profiles, or use cases, specific to that domain.
Examples of IHE Domains include:
Each domain develops and maintains its own set of Technical Framework documents which define the IHE standard for that domain (including the profiles in full detail). IHE Technical Frameworks are recognized by many vendors as the interoperability standard by which health information is exchanged.
The IHE Patient Care Devices (PCD) domain is the one that applies to medical device connectivity and integration. The PCD domain addresses the exchange of information between medical devices – including vital signs monitors, ventilators, infusion pumps, and anesthesia workstations – and clinical information systems. It is sponsored by the American College of Clinical Engineering (ACCE), the Health Information Management Systems Society (HIMSS), and the Association for the Advancement of Medical Instrumentation (AAMI).
What is an IHE Integration Statement?
Every year, IHE hosts Connectathon, a live event attended by all major medical device vendors, where vendors test interoperability between systems. Vendors that pass are given the opportunity to produce an IHE Integration Statement for their product that describes its conformance with IHE interoperability standards. The IHE Integration Statement is published on the IHE Product Registry.
The most important parts of the IHE Integration Statement are the “Integration Profile” and “Actor” columns. (“Actor” refers to the role that a device or system performs.)
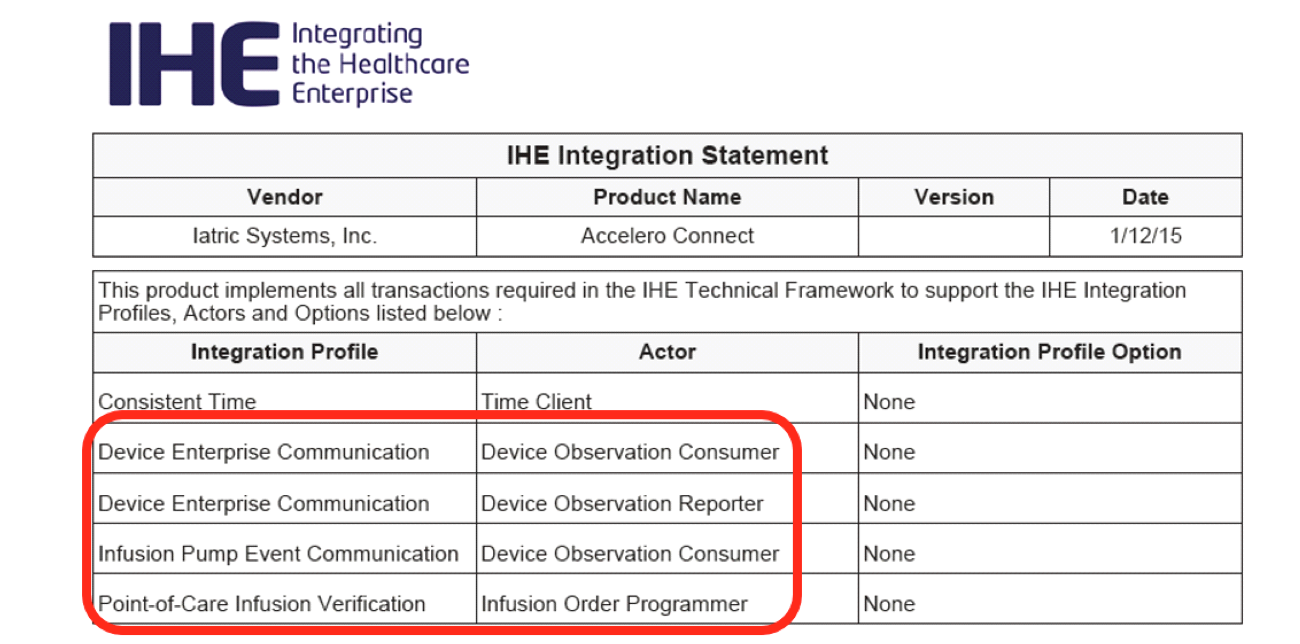
Here is what the above Integration Profile / Actor combinations mean in layman’s terms:
Iatric Systems Accelero Connect IHE Integration Statement verifies that Accelero Connect can communicate with any medical device manufacturer and/or EHR that complies with IHE Technical Frameworks.
{{cta('70a9d4fd-234b-48d3-a7f3-3d6509ff4142','justifycenter')}}